OTITIS EXTERNA (OE) AFFECTS 1 IN 5 DOGS
IN THEIR LIFETIME 1
The pain of OE and stress of at-home treatments can affect the quality of life of dogs
and their owners.
It can:2,3
- Affect dogs’ sleep and playing behaviours
- Lead to physical and emotional disturbances in owners
- Impair dog-owner relationships
What do vets recommend and pet owners prefer?
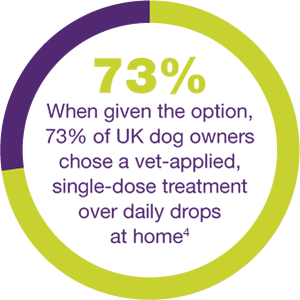

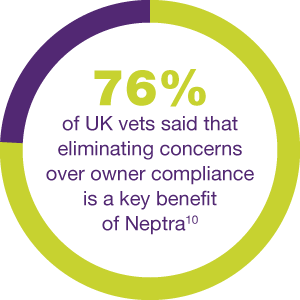
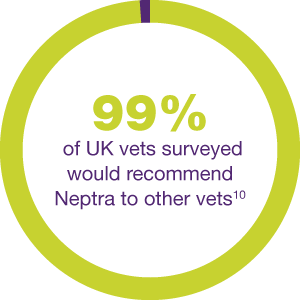




SOLVE EVERYDAY OE TREATMENT WITH JUST ONE DOSE

Early, effective vet-administered treatment can be key to successful outcomes. Neptra, administered in clinic, provides a full course of treatment in just one dose!
Avoid the uncertainty and ongoing stress of repeat at-home treatment with Neptra
- The first ONE-dose treatment for OE
- Vet-administered to guarantee 100% compliance
- Flexible recheck to suit you, the patient and the owner
THE ONLY 1-DOSE TREATMENT WITH FLORFENICOL – WHEN INDICATED, FLORFENICOL IS A 1ST-LINE ANTIBIOTIC; SEE THE PROTECT ME GUIDELINES*6
Neptra offers a combination of 3 active ingredients that work together against the most common pathogens associated with OE.
Anti-inflammatory

Antibiotic
Anti-fungal
GIVE DOGS RELIEF FROM THE PAIN AND DISCOMFORT OF EVERYDAY OE 5,8
With its unique combination of active ingredients, Neptra's 1-dose solution:
- Effectively treats up to 94% of routine canine OE cases**8
- Is licensed for the treatment of the most common pathogens of OE cases (Staphylococcus pseudintermedius and Malassezia pachydermatis)
- Provides lasting efficacy after one application, with continuous clinical improvement until day 289

Neptra treats the pain and inflammation of OE effectively, offering relief without multiple treatments.

Neptra can relieve stress and help protect the bond they have with their pets.

Neptra can give you confidence that the job has been done, effectively, there and then.










