GALLIPRANT, THE #1 PIPRANT1 CAN BE
USED TO DETECT AND TREAT PAIN AT
THE START OF A DOG’S OA JOURNEY.2–4
Osteoarthritis has traditionally been thought of a disease of older dogs, with the average
age of
OA
diagnosis being 10.5 years.5
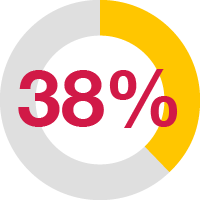
The first comprehensive, prospective OA evaluation study in younger dogs showed:6

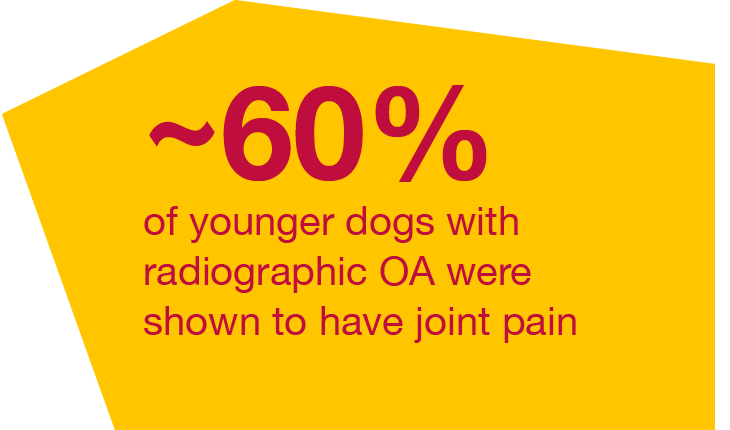
 123 dogs from single, first opinion/primary care enrolled from 8 months to 4 years of age and ≥3.6 kg
body weight.
Uncontrolled pain starts the cycle of decline for dogs with OA7
123 dogs from single, first opinion/primary care enrolled from 8 months to 4 years of age and ≥3.6 kg
body weight.
Uncontrolled pain starts the cycle of decline for dogs with OA7
Treating OA pain at earlier stages can improve the long-term outlook for most dogs.7 And recognition
of
subtle changes in a dog's discomfort, including changes in gait, mobility, energy and behaviour is key for
early OA detection.4
Pain management must be part of the OA discussion at the time of diagnosis.8
GET DOGS BACK TO THEIR PLAYFUL
SELVES WITH THE 1ST AND
ONLY PIPRANT THAT PRECISELY TARGETS CANINE OA PAIN9,10
DISCOVER MORE
Get the full details, videos and resources by logging into MyElanco.
Not registered yet? Don't miss out, see the benefits
of MyElanco.
Osteoarthritis has traditionally been thought of a disease of older dogs, with the average age of OA diagnosis being 10.5 years.5

The first comprehensive, prospective OA evaluation study in younger dogs showed:6



Treating OA pain at earlier stages can improve the long-term outlook for most dogs.7 And recognition of subtle changes in a dog's discomfort, including changes in gait, mobility, energy and behaviour is key for early OA detection.4
Pain management must be part of the OA discussion at the time of diagnosis.8
GET DOGS BACK TO THEIR PLAYFUL
SELVES WITH THE 1ST AND
ONLY PIPRANT THAT PRECISELY TARGETS CANINE OA PAIN9,10
DISCOVER MORE
Get the full details, videos and resources by logging into MyElanco.
Not registered yet? Don't miss out, see the benefits of MyElanco.











